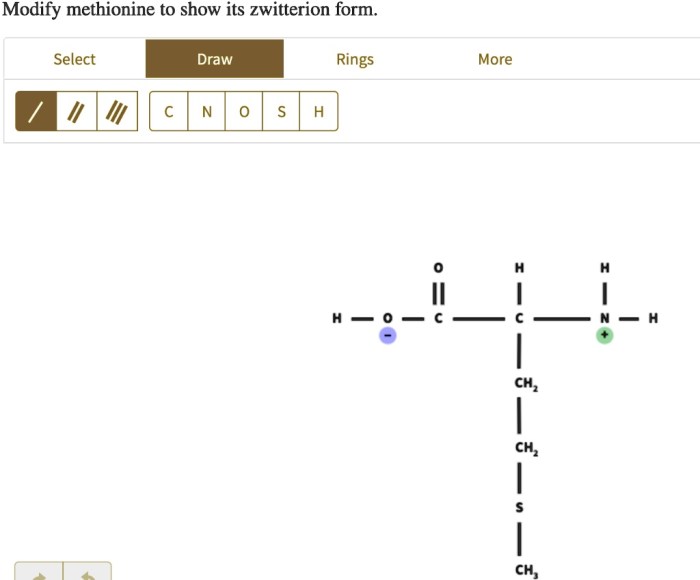
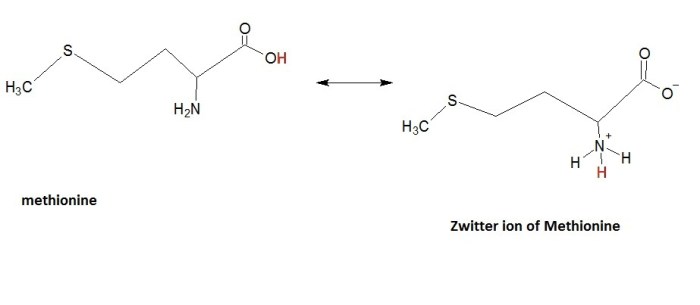
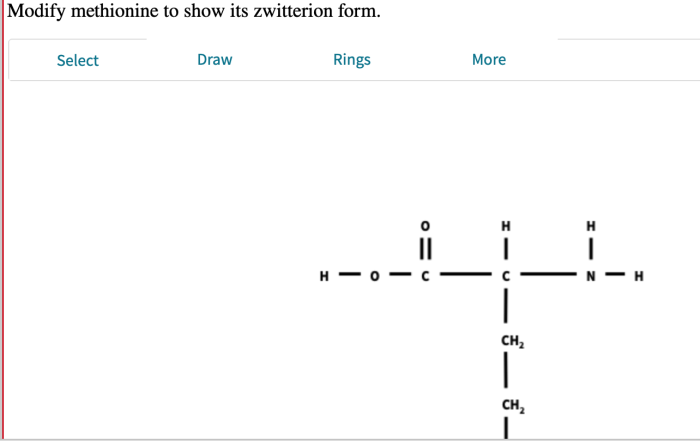
Modify methionine to show its zwitterion – Methionine, an essential amino acid, plays a crucial role in protein synthesis and exhibits a unique property of forming zwitterions. This article delves into the chemical structure, zwitterion formation, modification methods, applications, and characterization techniques of methionine, providing a comprehensive understanding of its significance in biochemistry and beyond.
Methionine Structure and Properties
Methionine is an essential amino acid with a unique structure and important role in protein synthesis. Its chemical structure consists of a central carbon atom bonded to an amino group, a carboxyl group, a methyl group, and a thioether side chain containing a sulfur atom.
The sulfur atom in methionine’s side chain gives it unique properties, including the ability to form covalent bonds with other molecules.
Zwitterion Formation
In aqueous solutions, methionine can exist as a zwitterion, a molecule with both positive and negative charges. The amino group becomes positively charged (NH3+) while the carboxyl group becomes negatively charged (COO-), resulting in a net neutral charge. The formation of zwitterions is influenced by pH, with the zwitterionic form predominating at neutral pH.
Methods to Modify Methionine
Chemical modifications of methionine can alter its properties and expand its applications. Common methods include:
- Oxidation: Converting the sulfur atom to a sulfoxide or sulfone.
- Alkylation: Adding alkyl groups to the sulfur atom.
- Acylation: Adding acyl groups to the amino or carboxyl groups.
These modifications can enhance solubility, stability, or reactivity of methionine.
Applications of Modified Methionine
Modified methionine finds applications in various fields, including:
- Pharmaceutical research: As building blocks for drug synthesis and as potential therapeutic agents.
- Biotechnology: In protein engineering and enzyme modification.
- Material science: As components of biomaterials and functional materials.
Spectroscopic Techniques for Characterization, Modify methionine to show its zwitterion
Spectroscopic techniques provide valuable insights into the structure and properties of modified methionine.
- Nuclear magnetic resonance (NMR): Determines molecular structure and dynamics.
- Mass spectrometry: Identifies molecular mass and composition.
- Ultraviolet-visible (UV-Vis) spectroscopy: Analyzes electronic transitions and chromophores.
These techniques help characterize modified methionine’s structure, conformation, and reactivity.
Computational Modeling
Computational modeling complements experimental techniques in understanding methionine modifications. Molecular dynamics simulations provide insights into the dynamic behavior of modified methionine in different environments. Quantum chemical calculations predict electronic structures and reaction pathways, aiding in the design of new modified methionine derivatives.
FAQ Guide: Modify Methionine To Show Its Zwitterion
What is the significance of methionine’s zwitterionic nature?
Methionine’s zwitterionic nature plays a crucial role in its solubility, protein interactions, and enzyme catalysis. It allows methionine to exist in both positively and negatively charged states, enhancing its ability to participate in various biochemical processes.
How can methionine be modified chemically?
Methionine can be modified using various chemical methods, such as alkylation, acylation, and oxidation. These modifications alter its side chain properties, affecting its solubility, reactivity, and biological activity.
What are the applications of modified methionine?
Modified methionine has applications in pharmaceutical research, where it can be used to develop drugs with improved stability, bioavailability, and therapeutic efficacy. It also finds use in protein engineering and biomaterial design.